The Botanical Register, vol. X (1824), note 784.
Edwards's Botanical Register, vol. XXX1 (1845), note 36 (The Sections of Epidendrum).

Epidendrum ciliare L.
| publié dans | Systema Naturae, Editio Decima: 1246. 1759. |
| synonymes : | Auliza ciliaris (L.) Salisb. Transactions of the Horticultural Society of London 1: 294. 1812. |
| Coilostylis emarginata Raf. Flora Telluriana 4: 37. 1838. |
|
| Epidendrum ciliare var. cuspidatum (G.
Lodd.) Lindl. Folia Orchidacea. Epidendrum 30. 1853. |
|
| Epidendrum ciliare var. minor hort. ex Stein Orchideenbuch 226. 1892. |
|
| Epidendrum ciliare var. squamatum Schnee Revista de la Facultad de Agronomía (Maracay) 1: 206. 1953. |
|
| Epidendrum ciliare var. viscidum
(Lindl.) Lindl. Folia Orchidacea. Epidendrum 30. 1853. |
|
| Epidendrum cuspidatum G. Lodd. Botanical Cabinet; consisting of coloured delineations . . 1: t. 10. 1816. |
|
| Epidendrum cuspidatum var. brachysepalum Rchb. f. Linnaea 19: 372. 1847 |
|
| Epidendrum luteum Planch. Hortus Donatensis 4: 165. 1858. |
|
| Epidendrum viscidum Lindl. Edwards's Botanical Register 26: misc. 81. 1840. |
|
| Phaedrosanthus ciliaris (L.) Kuntze Lexikon Generum Phanerogamarum 429. 1904. |
documents d'archives
|
The Botanical Register, vol. X (1824), note 784. Edwards's Botanical Register, vol. XXX1 (1845), note 36 (The Sections of Epidendrum). |

|
bibliographie sur l'espèce
Ames, O. & D. S. Correll, 1952, Orchids of Guatemala, Fieldiana, Botany, 26(1): i-xiii, 1-395. Balick, M. J., M. H. Nee & D. E. Atha, 2000, Checklist of the vascular plants of Belize, Memoirs of the New York Botanical Garden, 85: i-ix, 1-246. Brako, L. & J. L. Zarucchi, 1993, Catalogue of the Flowering Plants and Gymnosperms of Peru, Monographs in Systematic Botany from the Missouri Botanical Garden, 45: i-xl, 1-1286. Breedlove, D. E., 1986, Flora de Chiapas, Listados Florísticos de México, 4: i-v, 1-246. Carnevali F., G., J.L. Tapia-Muñoz, R. Jiménez-Machorro, L. Sánchez-Saldaña, L. Ibarra-González, I.M. Ramírez & M.P. Gómez, 2001, Notes on the flora of the Yucatan Peninsula II: a synopsis of the orchid flora of the Mexican Yucatan Peninsula and a tentative checklist of the Orchidaceae of the Yucatan Peninsula biotic province, Harvard Papers in Botany, 5(2): 383-466. Correll, D. S., 1965, Supplement to the Orchids of Guatemala (and British Honduras), Fieldiana, Botany, 31(7): 177-221. Cowan, C. P., 1983, Flora de Tabasco, Listados Florísticos de México, 1: 1-123. Dodson, C. H. & P. M. Dodson, 1984, Orchids of Ecuador, Icones Plantarum Tropicarum, 10: 901-1000. Dodson, C. H., 2001, Dresslerella-Lepanthes, Native Ecuadorian Orchids, 2 : 219-419. Hamer, F., 1982, Orchids of Nicaragua. Part 2, Icones Plantarum Tropicarum, Fasc. 8: 701-800. Hamer, F., 1988, Orchids of Central America, Selbyana, 10 (suppl.): 1-430. Jørgensen, P. M. & S. León-Yánez (eds.), 1999, Catalogue of the vascular plants of Ecuador, Monographs in Systematic Botany from the Missouri Botanical Garden, 75: i-viii, 1-1182. Kirsch F., 1982, Differentes formes de l' Epidendrum ciliare L. en
Martinique, Orchidophile 13. (50): 11-16. Martínez, E., M. Sousa S. & C. H. Ramos Álvarez, 2001, Región de Calakmul, Campeche, Listados Florísticos de México, 22: 1-55. McLeish, I., N. R. Pearce & B. R. Adams, 1995, Native Orchids of Belize, 1-278. 7. Molina R., A., 1975, Enumeración de las plantas de Honduras, Ceiba, 19(1): 1-118.
Pasetti M., 1996, La specie: Epidendrum ciliare L., Orchis no.110: 12-13. Schweinfurth, C., 1959, Orchidaceae, Orchids of Peru, Fieldiana, Botany, 30(2): 261-531. Stevens, W. D., C. Ulloa U., A. Pool & O. M. Montiel, 2001, Flora de Nicaragua, Monographs in Systematic Botany from the Missouri Botanical Garden, 85: i-xlii, 1-2666. Vasquez C., R. & P. L. Ibisch, 2004, Orquídeas de Bolivia: Diversidad y Estado de Conservación: Vol. II Laeliinae -- Polystachyinae -- Sobraliinae, con actualización y complementación de Pleurothallidinae, 1-649. Anonymous, 1986, List-Based Record, Soil Conservation Service, U.S.D.A. |
données de collecte pour la Colombie
|
localité |
collecteur | numéro de collecte | herbrarium | notes de collecte |
| Antioquia, Barbosa | M. Ospina | 192 | JAUM | "flores blancas". |
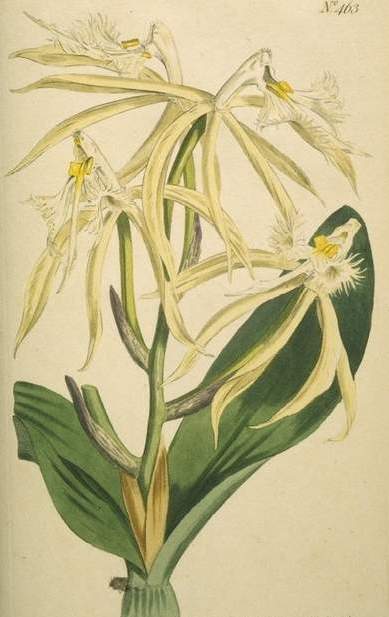 |
source de l'illustration : Curtis's
Botanical Magazine, 1799, volume 13, planche 463.
illustrateur : Sansum S. Edwards |
|
|
 |
|
|
source de l'illustration : |
Les Liliacées, 1802-1816, planche 82 - gravure en pointillé, imprimée en couleurs, finition manuelle. | |
|
illustrateur : |
Pierre-Joseph Redouté | |
fragrance et pollinisation de l'espèce
|
"Ackerman and Montalvo (1990) found proof for ... pollinator in Puerto Rico. ... The hawkmoth Pseudosphinx tetrio was a pollinator in one of the populations studied." An atlas of orchid pollination, America, Africa, Asia and Australia, p. 121. |
|
"as twilight approaches, they start emitting a very appealing ‘white-floral’ scent, accompanied during the first few hours by an astringent, grapefruit-like note. This is attributable principally to 2-methylbutyr-aldoxime and realted compounds, which are characteristic of night-scented flowering plants... its sent can also emphasize different aspects, depending on the origin of the plant." Kaiser, R. The Scent of Orchids, Olfactory and Chemical Investigations [traduction de Vom Duft der Orchideen - Ölfaktorische und Chemische Untersuchungen]; Elsevier, Amsterdam / Editiones Roche, F. Hoffmann La Roche:AG Bâle, 1993, pp. 90-91. |
| "A highly variable floral scent composition was found
among non-rewarding flowers of the beetle-pollinated Magnolia kobus
DC. (Azuma, Toyota, and Asakawa, 2001 ) and the moth-pollinated orchid Epidendrum
ciliare L. (Moya and Ackerman, 1993 ). In female-stage flowers
of M. kobus, no single floral scent compound was found in all
individuals. Furthermore, individuals or whole populations were
characterized either by oxygenated terpenoids and benzenoids, N-compounds,
and benzenoids, or by terpenoid hydrocarbons combined with benzenoids and/or
oxygenated terpenoids. In E. ciliare,
the similarity in floral scent composition among four populations was
likewise very low, ranging from 12 to 21%. Pollination in both species is
suggested to rely partly or wholly on deception. However, in M. kobus
the large variation is suggested to reflect low importance of scent
compared to visual cues in attracting pollinators, while the deception in E.
ciliare is suggested to rely on disruption of the learning
processes in the exploratory behavior of food-searching, naïve moths."
Jette T. Knudsen, Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the western Amazon, American Journal of Botany, (2002) 89:1772-1778. |